Holy upheaval, Batman. Welcome to my novel: I’ve been working on this piece on and off since December. It was originally slotted for December 5, but the Pfizer delay followed by Omicron news sort of jumped ahead of things, and then I intended it for February 5, but decided to wait until we had some more information about Pfizer’s EUA submission for the pediatric vaccine for kids under 5, which was supposed to be in front of the FDA on the 15th. Then on Friday, Pfizer said “no, wait, actually” and pulled the application, so we’re back on the March/April timeline for kids under 5 to be eligible for vaccination. Quite the whirlwind! It’s meant a lot of substantial revisions on this letter.
I’m also nervous about putting this one out there, because the conversation about kids, Covid, and pediatric vaccines can get so absolutely vitriolic, and because it’s critically important that I get it right. I want to be sure that everything I include here is information I can stand firmly behind, and that if it changes later, at least I’m sharing a reliable collection of the information I’m working with now. I’ve carefully read everything included here, including some amount of commentary on it, to make sure it says what I think it says with a level of confidence I’m comfortable with. Which isn’t to say I don’t always try to do that; I do. But this particular topic requires an extra level of attention.
So first, a caveat: one of the loudest ongoing kid-related conversations during all of this has been about whether schools should be open, in which capacities, and with which mitigation measures. I want to be open here that I am a stay-at-home, homeschooling parent, and have intended to be since before my kid was born, so while I am on top of the debate about schools and the inequitable consequences of closures1, my personal stake in the question is limited, and I’m not going to address it in this letter. That said, I have a four-year-old kid currently ineligible for vaccination, all her peers are also ineligible and vulnerable, and even homeschooling parents typically have much more access to community learning opportunities than we’ve had in the past two years. We want to go out and be around other kids as much as school kids do! It’s important to remember that, while when cases are low, schools can be made safe, or at least safer2, there may be times when the safety of schools as part of the community is severely compromised.
Ok, So, What the *%&# Just Happened?
in December, Pfizer decided not to submit the data on their trial for vaccines in kids under 5, because while they saw no safety concerns in that age group, they also didn’t see the immune response they’d hoped for in kids 2-4 years old. Instead, they were planning to test a third dose in that age group, with data submission to follow. Due to some bureaucratic stuff surrounding the study design, they also can’t just submit for the younger kids, even though it was safe and effective for them. I don’t actually know the specific reasons for that, only that it seems to be the case.
Then in January, the FDA requested that Pfizer submit the data they’d collected in the first part of the trials so it could be considered for emergency use authorization. This was a bit surprising, because that’s not usually how these things work. The assumption about the FDA’s request was that, given the increased risk to young kids from the Omicron variant (see below), they wanted to see Pfizer’s data on the under-fives so the FDA could be part of the risk-benefit analysis based on that data. Presumably, Pfizer knows how effective their vaccine was against Delta, and has some data on how effective it is against Omicron. It seems like the FDA wanted to know that information and make a decision about whether the data showed enough protection to make it worth it when weighed against Omicron.
What we had expected was that the data would be released to the public today, with a meeting scheduled on Monday for the FDA to make a decision about whether kids could start their vaccination now and add a third dose later, when the trials are finished. Instead, this afternoon, Pfizer withdrew their application3. This means that not only will the FDA not be deciding on vaccines for kids under five next week, the data they would have been considering is also not available for us to review. So we don’t know, exactly, what went into any of these decisions.
Without the early EUA decision, we expect to see Moderna’s submission for kids under six in March, and Pfizer’s researchers are now trialing a third dose of this series in the 2-4 year old group to see if it achieves a higher level of protection for them, with data expected to be ready for the FDA in April. It seems likely that this age group will have three doses for their “primary series,” unlike other groups which still have a two-dose primary series; that is, there will be three doses by default, rather than two doses and a booster (some have argued that everyone should be starting with a 3-dose series, but that’s currently an open debate). Younger kids, whose protection from the two-dose series was higher, may or may not need the third dose. It’s also possible, though on a much longer timeline, that 3- and 4-year olds may end up getting the 10mcg dose currently offered to kids 5-11.
Historically, the major manufacturers have submitted their data to Health Canada at roughly the same time they submit to the FDA. Canada’s approval process for Covid-related applications is different4, but based on past approvals, we expect a roughly similar timeline.
So that’s today’s news. But that’s not actually why I’m here! I want to talk about vaccines more generally, and to do that, we have to consider the risk/benefit question.
The major debate about pediatric vaccines is whether they’re necessary enough and safe enough; that is, whether the risk of Covid for these kids warrants the expense and potential risk of mass vaccination. So I’m going to start by summarizing the risk side of that equation, and then get into the vaccines themselves.
How Serious is the Covid-19 Disease in Children?
I want to be able to give you a clear answer on this, but to be honest, I can’t. I can’t know your household’s individual risk factors, and I am very aware that my household has a much lower risk tolerance than average. So I’m going to go through what I know and hope it helps guide your decisions, even if they’re different from mine.
Early in the pandemic, many parents (myself included) were relieved to find out that, for the most part, serious illness seemed to be sparing kids. When they did get sick, it tended to be brief and mild (and to be precise, it wasn’t just “clinically mild,” in that they weren’t being hospitalized, but typically it was genuinely cold-mild, with extremely rare complications). That was the case with Wild Type Covid-19, and if we were still dealing with Wild Type Covid, we might be having a different conversation, although the question of long Covid would still be an important one5.
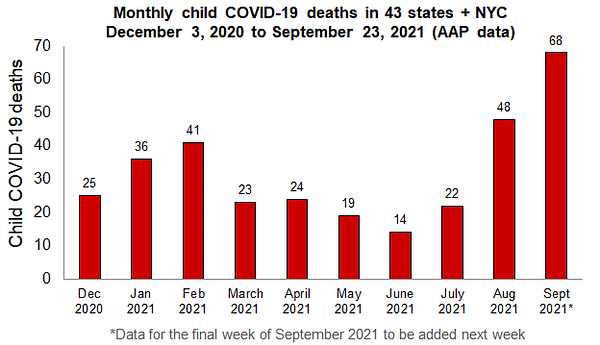
But then we got variants. And while the earliest variants held the line for kids, by the time Delta came around, the risks had grown6. While Omicron doesn’t seem to be more dangerous for individual kids than Delta7, it’s still more dangerous than Wild Type, and so much more contagious that the numbers of kids who get seriously sick or die are much higher now than they’ve ever been8. As things stand, more than half of the total number of severe infections and deaths in children have happened in the most recent eight weeks of the pandemic9. While the proportional numbers are still low10, the number of sick kids is alarming, meaning that the combination of a disease that seems to impact children more and lower adherence to community protection measures like masking and social distancing means that kids, especially unvaccinated kids, are at greater risk right now than at any other time during the pandemic.
For the average kid, that relative risk may not feel like a big deal, and it’s true that most kids who get Covid will still get through it without complications. But it’s a lot harder to avoid Covid altogether now, and every infection is a gamble. And remember: we don’t know what the long-term impact on kids’ lives will be if they get sick, and we know that things like permanent cardiovascular disease are on the table.11 We also know that the line between “healthy” and “high risk” is a blurry one, and kids whose conditions are otherwise well-controlled may be facing worse odds. Finally, we know that when kids get sick, so do the people around them12, and as of October, more than 140,000 kids had lost a parent or caregiver13.
Is it really worse than…?
Many parents and pundits argue that Covid infections in kids are more or less like other childhood infections, like flu and RSV, but even accounting for the lower overall incidence of those diseases during the pandemic and the fact that we also work hard to protect the youngest kids from those illnesses, the total hospitalization and death rates in young children are elevated well above pre-pandemic expectations.
What all this means, ultimately, is that the total risk of serious illness, permanent disability, and death in children is higher than it was in 2019. For me, that’s enough, but it’s a understandable to have questions about what that risk level means for your specific child. For me, the overall risk level of an immune-naive infection (that is, infection before vaccination) is higher than I want my kid to have to live with.
Ok, So How are the Vaccines Doing?
Of the vaccines available in the US and Canada, Pfizer is currently the furthest along in the process, with Moderna close on their heels and several others well into the process. Because mRNA vaccines are currently the preferred option in North America overall and the only ones currently approved for anybody under 18 in those countries, for our purposes I’ll be talking about just Pfizer and Moderna.
All of our current vaccines are running age step-down trials, which means they test in adults first, then in age groups descending down to infants, one group at a time. The first step for both groups has been establishing dosage, with a key difference between the two: Pfizer has been using a model that starts by seeking the lowest effective dose and then increasing dosage to check for side effects, while Moderna has gone the opposite direction by starting with the highest safe dose and decreasing until they see reduction in efficacy. In most cases, this has resulted in slightly higher effectiveness in Moderna vaccines, but with slightly higher rates of side effects. In general, Pfizer sees slightly fewer side effects, with slightly lower effectiveness. This has held true in most age groups, but there’s an open question as to whether Moderna’s strategy may have better results for the youngest kids, where it’s possible the difference in dosage may lead to a bigger difference between outcomes.
So far, here’s what we know about each age group’s risks and benefits from the vaccines:
Kids 12-18 get a 30mcg dose of Pfizer or 100mcg of Moderna, the same dose as adults. It has been very effective, and the waning protection seen by adults took longer in this age group. While boosters are starting to become available for them, it’s slow going, both because of the risk profile and because their original series is still working pretty well. There were some safety concerns for this age group; they saw a slightly higher rate of myocarditis and pericarditis than in adults, although it’s still extremely rare and much less severe than the versions caused by Covid infection14. Prior to Omicron, vaccines for this age group were rated at 95-100% effective against Covid. It has dropped a bit, but wow!
Kids 5-11 get a 10mcg dose of Pfizer (Moderna is still doing trials for this age group, but expects to offer a 50mcg dose based on my latest information). This dosage has been both very effective and very safe15, with fewer than a dozen reports of serious complications (like myocarditis and pericarditis) in millions of vaccinated kids.
At the moment, kids 2-4 years old are expecting to get a 3mcg dose of Pfizer or a 25mcg dose of Moderna. There have been no safety issues reported in the trials, but in December, Pfizer announced that this age group wasn’t mounting the expected immune response from two doses. Right now, Pfizer is testing a third dose for this group. Moderna expects to submit data on their trial for this group in March.
Kids 6-24 months also expect to get a 3mcg dose of Pfizer or a 25mcg dose of Moderna. Again, there are no safety signals for this age group, and in the Pfizer trials, the littlest kids mounted an immune response comparable to people 16-24 years old, the “goalpost” group.
What Will I Do?
My child will be vaccinated as soon as possible with whatever vaccine is first available to her, and until then, we will take all available measures to avoid her being exposed. I can say this knowing we were also willing to participate in trials (some of my information about the dosages for the littlest kids is from the informed consent paperwork we were given by Moderna): I’m extremely confident in the safety of these vaccines. While my daughter’s individual risk from Covid may be low, it’s never going to be zero, and her best chances of good outcomes are if she’s vaccinated before she’s exposed to the virus.
Addendum: We Don’t Talk About VAERS
VAERS, or the Vaccine Adverse Event Reporting System, pops up in the news a lot, especially with regard to Covid vaccine skepticism. I want to give a little rundown of what it is and why I don’t take it too seriously by itself.
First and foremost: VAERS is a self-reported dumping ground, and it’s especially vulnerable to manipulation when people are highly motivated to undermine vaccines. Any symptom anybody experiences following vaccination is reportable, without any evaluation of whether or not the symptom is actually associated with the vaccine in any way. So when people pull VAERS reports directly, they get a lot of junk data.
What VAERS is good for is pulling together signals. If a lot of people are reporting the same symptom following the same vaccine, doctors and scientists can investigate that signal. They can consider the profiles of the reports and even do follow up investigative diagnostics with those who report the symptoms. Signals like VAERS are how we’ve identified and evaluated the risks of things like myocarditis after mRNA vaccines and Vaccine-Induced Prothrombotic Immune Thrombocytopenia (VIPIT) following vaccination with the AstraZeneca and Janssen formulas. Those signals allowed doctors to determine the risk factors and compare them to the risks posed by Covid infection, and make recommendations about vaccination. Without those assessments, the VAERS data doesn’t help with decision-making. Having reviewed both the VAERS data and the ongoing evolution of expert analysis, I’m confident in the vaccine safety recommendations made by bodies like the FDA and NACI.
“The Kids Left Behind.” The Local, 31 Jan. 2022, https://thelocal.to/the-kids-left-behind/.
February 3, Michelle HolmesUpdated, et al. “We Need to School Ourselves as to What Safe In-Person Education Looks like - The Boston Globe.” BostonGlobe.Com, https://www.bostonglobe.com/2022/02/03/opinion/we-need-school-ourselves-what-safe-in-person-education-looks-like/. Accessed 10 Feb. 2022.
Pfizer Withdraws Application:
Tracy, Prof. “News about Vaccine for Young Kids.” TikTok, February 11, 2022. https://www.tiktok.com/@scitimewithtracy/video/7063536758323023150?_t=8PnNCNVBlnq&_r=1.
LaFraniere, Sharon, and Noah Weiland. “Covid Live Updates: In Reversal, F.D.A. Delays Decision on Shots for Children Under 5.” The New York Times, February 11, 2022, sec. World. https://www.nytimes.com/live/2022/02/11/world/covid-19-tests-cases-vaccine.
Canada, Health. Drug and Vaccine Authorizations for COVID-19: Overview. 14 Oct. 2020, https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization.html.
“Long COVID and Kids: More Research Is Urgently Needed.” Nature 602, no. 7896 (February 8, 2022): 183–183. https://doi.org/10.1038/d41586-022-00334-w.
Parcha, Vibhu, Katherine S. Booker, Rajat Kalra, Seth Kuranz, Lorenzo Berra, Garima Arora, and Pankaj Arora. “A Retrospective Cohort Study of 12,306 Pediatric COVID-19 Patients in the United States.” Scientific Reports 11, no. 1 (May 13, 2021): 10231. https://doi.org/10.1038/s41598-021-89553-1.
Kozlov, Max. “Does Omicron Hit Kids Harder? Scientists Are Trying to Find Out.” Nature, February 4, 2022. https://www.nature.com/articles/d41586-022-00309-x.
Yamey, Gavin. “Why Do I Support Child Covid-19 Vax? Short 🧵,” February 2, 2022.
Julia Raifman. “This Month, a Record Number of US Children Died of COVID-19. And the Month Is Not over. Children Rely on Adults and Policymakers to Protect Them. ➡️ Community Mask Policies ➡️ Vaccine Delivery in Schools + Neighborhoods, to Kids + Everyone Thank You @AAPNews for Tracking Https://T.Co/XH6NgdiMbf.” Tweet. @JuliaRaifman (blog), September 27, 2021.
Mayo Clinic. “How COVID-19 (Coronavirus) Affects Babies and Children.” Accessed October 2, 2021. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
“Heart-Disease Risk Soars after COVID — Even with a Mild Case.” Nature, February 10, 2022. https://doi.org/10.1038/d41586-022-00403-0.
News, Susie Flaherty MGH, and Public Affairs. “Study Confirms Kids as Spreaders of COVID-19 and Emerging Variants.” Harvard Gazette (blog), October 14, 2021. https://news.harvard.edu/gazette/story/2021/10/study-confirms-kids-as-spreaders-of-covid-19-and-emerging-variants/.
Centers for Disease Control and Prevention. “The Hidden U.S. COVID-19 Pandemic: Orphaned Children – More than 140,000 U.S. Children Lost a Primary or Secondary Caregiver Due to the COVID-19 Pandemic,” October 7, 2021. https://www.cdc.gov/media/releases/2021/p1007-covid-19-orphaned-children.html.
Safety & Effectiveness: 12-18
Patone, Martina, Xue W. Mei, Lahiru Handunnetthi, Sharon Dixon, Francesco Zaccardi, Manu Shankar-Hari, Peter Watkinson, et al. “Risks of Myocarditis, Pericarditis, and Cardiac Arrhythmias Associated with COVID-19 Vaccination or SARS-CoV-2 Infection.” Nature Medicine, December 14, 2021, 1–13. https://doi.org/10.1038/s41591-021-01630-0.
“New Analysis Shows Benefits of Offering Two Doses of COVID-19 Vaccine to Children Aged 12-17 Clearly Outweigh Risks.” Accessed October 3, 2021. https://www.rsm.ac.uk/media-releases/2021/new-analysis-shows-benefits-of-offering-two-doses-of-covid-19-vaccine-to-children-aged-12-17-clearly-outweigh-risks/.
Simone, Anthony, John Herald, Aiyu Chen, Neil Gulati, Albert Yuh-Jer Shen, Bruno Lewin, and Ming-Sum Lee. “Acute Myocarditis Following COVID-19 MRNA Vaccination in Adults Aged 18 Years or Older.” JAMA Internal Medicine, October 4, 2021. https://doi.org/10.1001/jamainternmed.2021.5511.
Heymans, Stephane, and Leslie T. Cooper. “Myocarditis after COVID-19 MRNA Vaccination: Clinical Observations and Potential Mechanisms.” Nature Reviews Cardiology 19, no. 2 (February 2022): 75–77. https://doi.org/10.1038/s41569-021-00662-w.
Safety & Effectiveness: 5-11
“Even More Safety and Effectiveness Data for COVID-19 Vaccines for Children | Johns Hopkins Bloomberg School of Public Health.” Accessed February 2, 2022. https://publichealth.jhu.edu/2022/even-more-safety-and-effectiveness-data-for-covid-19-vaccines-for-children.
Washington Post. “Low Dose of Pfizer-BioNTech Vaccine Is Safe and Effective in Children Ages 5 to 11, Companies’ Study Finds.” Accessed September 26, 2021. https://www.washingtonpost.com/health/2021/09/20/covid-vaccine-for-children/.


![[Citation Needed]](https://substackcdn.com/image/fetch/w_36,h_36,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fbucketeer-e05bbc84-baa3-437e-9518-adb32be77984.s3.amazonaws.com%2Fpublic%2Fimages%2F97161614-615c-4ecb-9659-79f8a54204f1_1280x1280.png)